Dengue Fever in South and Southeast Asia
Photo by Srivatsan Balaji on Unsplash
By Mikaela Daly Lubin
Published Winter 2023
Special thanks to Seth Ririe for editing and research contributions.
Viewpoints published by Ballard Brief are not necessarily endorsed by BYU or The Church of Jesus Christ of Latter-day Saints.
Summary+
Dengue fever is a mosquito-borne illness that impacts South and Southeast Asia at significant rates. It is carried primarily by the aedes aegypti mosquito, a species that thrives in warm, tropical climates. Features of dengue include excruciating pain and, in severe cases, internal bleeding, shock, and death. The spread of dengue in South and Southeast Asia is due to several factors, including advancements in transportation, poor preventative measures, poor sanitation, and subsequent standing water, which is an ideal breeding ground for mosquitoes. Dengue causes many negative consequences on individuals and communities it affects, including pain and suffering, lasting physiological effects, a significant economic burden on households and community systems, and poor mental health. Two innovative practices to combat the spread of dengue are sticky traps that catch mosquitoes and identify whether they carry dengue and improved fluid management for those already infected.
Key Takeaways+
- Dengue fever is a disease transmitted by aedes aegypti mosquitoes that can result in significant pain and, in some cases, severe illness and death.
- Dengue cases are exceptionally high in South and Southeast Asia. Inhabitants of Southeast Asia are 18 times more likely to contract dengue than their American counterparts.158
- Globalization and advancements in transportation have made the spread of dengue across continents and regions increase in recent decades.
- Problems with waste management in South and Southeast Asia cause the buildup of stagnant water, which is an ideal breeding ground for dengue-carrying mosquitoes. A study found that in storm drains with stagnant water, 21% housed aedes aegypti mosquitoes, but when the water was removed, none were found. Traditional prevention strategies such as insecticides have become less effective due to mosquito resistance. One type of insecticide used in Malaysia was ineffective in killing 75% of aedes aegypti mosquitoes.
- Dengue places a significant burden on individuals’ physiological and mental health, as well as on individual and community economies.
- New strategies such as sticky traps that capture and screen mosquitoes for the presence of dengue and innovations in the treatment of infected individuals have demonstrated promising practices for future care.
Key Terms+
Disease Burden—The total, cumulative consequences of a defined disease or a range of harmful diseases with respect to disabilities in a community.1
Endemic—Restricted or peculiar to a locality or region.2
Hematocrit—The ratio of the volume of red blood cells to the total volume of blood as determined by the separation of red blood cells from the plasma, usually by centrifugation.3
Hemorrhagic fever—Any of a diverse group of virus diseases (such as Lassa fever and Ebola) that are usually transmitted by arthropods or rodents and are characterized by a sudden onset, fever, aching, bleeding in the internal organs, petechiae, and shock.4
Morbidity—The incidence of disease: the rate of illness (as in a specified population or group).5
Mortality—The death of large numbers (as of people or animals).6
Myalgia—Pain in one or more muscles.7
RNA—Any of various nucleic acids that contain ribose and uracil as structural components and are associated with the control of cellular chemical activities.8
Serotype—A group of intimately related microorganisms distinguished by a common set of antigens.9
Vaccine—A preparation of killed microorganisms, living attenuated organisms, or living fully virulent organisms that are administered to produce or artificially increase immunity to a particular disease.10
Vector—An organism (such as an insect) that transmits a pathogen.11
Virus—Any of a large group of submicroscopic infectious agents that are usually regarded as nonliving, extremely complex molecules that typically contain a protein coat surrounding an RNA or DNA core of genetic material but no semipermeable membrane, that is capable of growth and multiplication only in living cells, and that cause various important diseases in humans, animals, and plants.12
Context
Q: What is dengue fever?
A: Dengue fever, abbreviated as DENV, is a disease caused by an RNAAny of various nucleic acids that contain ribose and uracil as structural components and are associated with the control of cellular chemical activities.8 virusAny of a large group of submicroscopic infectious agents that are usually regarded as nonliving, extremely complex molecules that typically contain a protein coat surrounding an RNA or DNA core of genetic material but no semipermeable membrane, that is capable of growth and multiplication only in living cells, and that cause various important diseases in humans, animals, and plants.12 and transmitted by aedes aegypti mosquitoes.13 It is not only transmitted from mosquitoes to humans but can also be transmitted back to mosquitoes when a mosquito feeds on an infected host.14 As such, the closer people are to each other, the more rapidly the disease can be transmitted. Dengue fever is also referred to as “break bone fever”15 because of the pain it causes in the muscles, joints, and bones.16 Once infected with dengue, an individual has a 25% chance of presenting symptoms;17 however, asymptomatic individuals transmit 88% of all dengue cases.18 After being bitten by a mosquito, dengue has an incubation period of 5–7 days before symptoms appear.19 Symptoms are described as either “severe” or “less severe.” Less severe dengue is more common, with symptoms including rash, muscle and joint pain, pain behind the eyes, and nausea and vomiting.20 While these “less severe” symptoms usually subside within a week, they can be extremely painful. The less common form of dengue, severe dengue, is a leading cause of death in southeast Asia.21 About 5% of people who contract dengue will demonstrate severe symptoms, including hemorraghic feverAny of a diverse group of virus diseases (such as Lassa fever and Ebola) that are usually transmitted by arthropods or rodents and are characterized by a sudden onset, fever, aching, bleeding in the internal organs, petechiae, and shock.4, which will later be discussed in depth.22 However, one study in India found that about 35% of dengue cases are severe.23, 24
There are 4 serotypesA group of intimately related microorganisms distinguished by a common set of antigens.9 of dengue fever, which had all spread throughout Asia by the end of WWII. Serotypes denote different strands of the virusAny of a large group of submicroscopic infectious agents that are usually regarded as nonliving, extremely complex molecules that typically contain a protein coat surrounding an RNA or DNA core of genetic material but no semipermeable membrane, that is capable of growth and multiplication only in living cells, and that cause various important diseases in humans, animals, and plants.12 that are united by certain characteristics of their makeup.25 Current research indicates that infection with one serotypeA group of intimately related microorganisms distinguished by a common set of antigens.9 of dengue fever may leave the individual more susceptible to severe hemorraghic feverAny of a diverse group of virus diseases (such as Lassa fever and Ebola) that are usually transmitted by arthropods or rodents and are characterized by a sudden onset, fever, aching, bleeding in the internal organs, petechiae, and shock.4 when infected with a different serotype.26
Q: Where is dengue fever most prevalent?
A: Dengue fever occurs at high rates in tropical and subtropical areas of the world, including South and Southeast Asia.27 This area’s susceptibility to heavy rainfall and subsequent humidity makes it an ideal breeding ground for aegypti mosquitoes.28 In Southeast Asia and India, the average annual rainfall is between 39–118 inches.29 A 2013 study in India indicated that dengue incidence was worst in a coastal, humid city and after monsoon seasons, indicating that humid climate and rainfall may contribute to disease prevalence.30 Another study demonstrated that higher dengue transmission coincided with monsoon patterns in Sri Lanka.31
It is estimated that half of the world’s population is considered “at risk” for dengue by the World Health Organization.32 There are 390 million cases of DENV every year, with 96 million of those cases occurring in individuals who demonstrate symptoms of the disease.33 This leaves roughly 294 million cases that are asymptomatic. In 2022, 3 of the 4 countries with the highest rates of actually reported dengue were in South and Southeast Asia: Vietnam reported 145,536 cases, the Philippines reported 52,597, and Indonesia reported 68,903; the only country with higher dengue incidence is Brazil.34 Worldwide, it is estimated that 75% of the reported cases of dengue come from the continent of Asia and the islands of the Pacific, including Australia and New Zealand.35 Inhabitants of Southeast Asia are 18 times more likely to contract dengue than their American counterparts.36
However, the exact disease burdenThe total, cumulative consequences of a defined disease or a range of harmful diseases with respect to disabilities in a community.1 of dengue in southeast Asia remains unmeasured because measurement requires laboratory confirmation, and many people sick with dengue are not tested.37 Based on data from the Global Health Data Exchange, South Asia reported an age-standardized rate of 1740.79 per 100,000 people in 2019, with the highest rates being in India, Bangladesh, and Sri Lanka.38 Age-standardized rates are a statistical analysis tool that estimates what rates would have been if age were not a variable.39 In Southeast Asia, the age-standardized rate of dengue was 1153.57 per 100,000 people, with the highest rates being in Thailand, Vietnam, and Indonesia.40 In India, it was estimated, based on the age-standardized rate, that the actual rate of dengue was about 2,020 per 100,000 individuals.41 This year in India, there have only been 110,473 cases reported in total.42
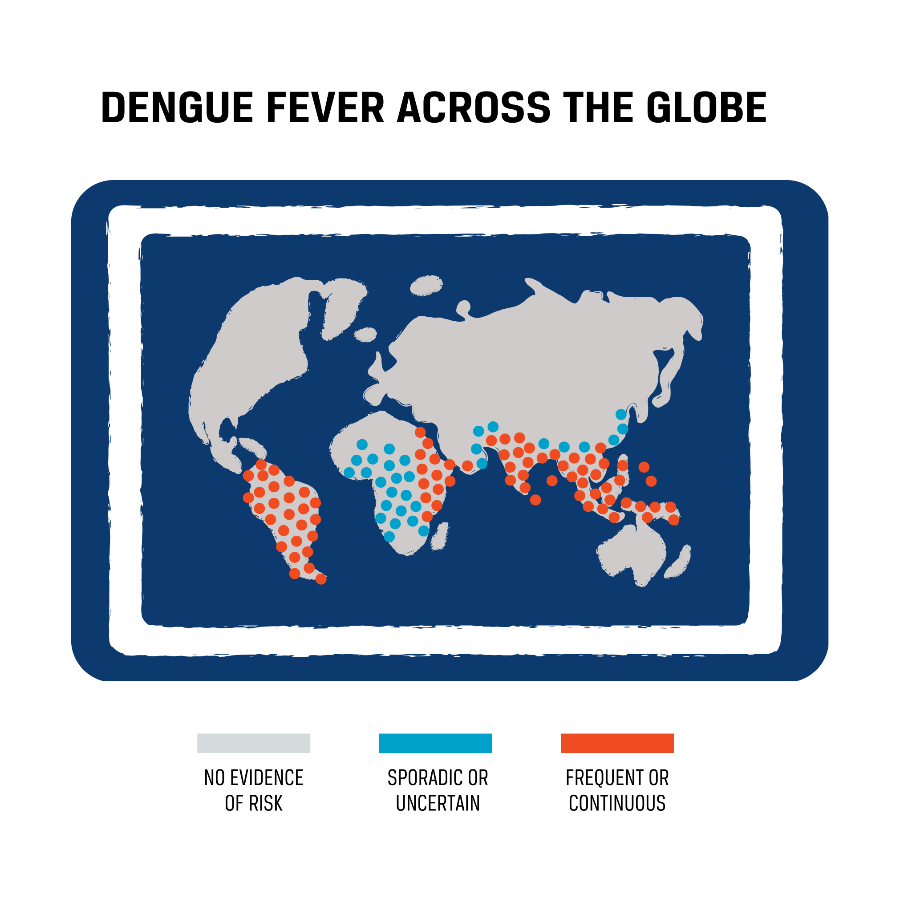
South and Southeast Asia are represented in the following maps.
The following map demonstrates where dengue incidence was most severe globally from January to August of 2022. As demonstrated by the map, almost the entire region of South and Southeast Asia have a frequent or contagious risk of dengue.
A 2015 study demonstrated that aedes aegypti mosquitoes have the highest distribution of occurrences in Taiwan, with 9,490 occurrences. The second was Brazil, with a little over half that.43 However, aedes aegypti mosquitoes (and the virusAny of a large group of submicroscopic infectious agents that are usually regarded as nonliving, extremely complex molecules that typically contain a protein coat surrounding an RNA or DNA core of genetic material but no semipermeable membrane, that is capable of growth and multiplication only in living cells, and that cause various important diseases in humans, animals, and plants.12) are spreading from these humid regions to more arid climates, making it an increasing global health threat, not just a problem of the tropics. Between the 1970s and 2019, dengue increased in incidence from being endemicRestricted or peculiar to a locality or region.2 in only 9 countries to 128 countries.44 It was on the World Health Organization’s list of threats to global health for 2019.45
Q: How has the prevalence of dengue fever changed over time?
A: The first records of dengue fever come from China in 992 CE,46 but the disease did not become a significant global health issue for over a millennium when dengue was relatively contained in endemicRestricted or peculiar to a locality or region.2 regions. Globalization beginning in the 17th century allowed for the disease to spread more rapidly as port cities around the world grew.47 The transmission was still more limited than it is today, with about 10–40 year gaps between major outbreaks, because the introduction of the pathogen to a new area depended on the much slower method of sailing ships.48 Prior to the 1940s, surges of dengue were less common and confined to coastal, tropical regions of the world.49 During this time, the populations living in urban areas in the south Asian region were not notably large.50 Because communities were smaller and isolated, dengue spread slowly from community to community.51 This is because, as discussed previously, dengue can be transmitted from mosquitoes to humans and back to mosquitoes again.
Advancements in transportation in the second half of the 20th century created new opportunities for dengue-carrying mosquitoes to move from place to place and thus increased rates of transmission. World War II contributed to the propagation of dengue; previously uninfected (and therefore susceptible) troops came in droves to the cities of Southeast and South Asia, presenting themselves as hosts for dengue. The more soldiers who were infected, the faster the disease spread. As the troops moved from one area to the next, now carrying the disease, dengue moved with them and magnified the number of cases in this region.52 WWII was especially problematic for the spread of dengue as it increased the circulation of different serotypesA group of intimately related microorganisms distinguished by a common set of antigens.9.53 This was a major turning point in the spread of dengue in South and Southeast Asia and created an environment ripe for mosquito reproduction. By the end of the war, many Asian nations were considered hyperendemic with dengue,54 meaning the disease was constantly occurring at high levels in this region from this time forward.55 In the decades following the war, several South and Southeast Asian nations, notably the Philippines, Thailand, Malaysia, and others, documented their first major outbreaks of severe dengue.56 Between the 1960s and 2008, the incidence of dengue increased globally 30-fold.57
Q: Who is most likely to be infected with dengue fever?
A: The demographics of those affected by dengue fever are broad due to the large population it affects, and there is a significant amount of discrepancy in the literature—especially over what age groups are most affected. One study in Thailand demonstrated that only 30–40% of positive cases are in individuals over 15 years old, with the other 60–70% of cases in individuals under 15. They noted that this was an increase in average age from previous Thailand reports. This may, in part, be attributed to adult tourists who contract the disease and seek treatment in the nation they are visiting.58 Another study indicated the most commonly infected individuals are males between the ages of 16–30.59 In a study on dengue in Faisalabad, Pakistan, 91% of all patients were adults.60, 61
There appears to be some discrepancy in the literature in regard to socioeconomic status as an influencing factor of dengue. Although traditionally thought to be a disease of poverty, a 2015 systematic review found limited evidence to support this claim.62
Studies differ on whether males or females have higher disease rates. Some studies demonstrate that women have higher rates, some indicate that men have higher rates, and some appear equal.63, 64, 65, 66 It is possible that the higher infection rate among males in some South and Southeast Asian countries may be attributed to cultural practices of women’s religious coverings and other social gender norms specific to these regions.67
Severe dengue appears to be more prevalent among children having had dengue previously, diabetes or kidney diseases, and early warning signs such as enlarged liver, abdominal pain, and decreased platelet count.68
For the purposes of this paper, statistics will not specify demographics such as gender or age. Most research done on different aspects of dengue fever discuss populations as a whole, not specific demographics other than geographic region.
Contributing Factors
Urbanization
Increasing urbanization in South and Southeast Asia has contributed to the spread of dengue through two main ways: globalization of the international market in conjunction with improvements to transportation and the buildup of stagnant water through poor waste management systems.
Globalization and Transportation
A major way that dengue fever has increased in prevalence in South and Southeast Asia is through globalization and subsequent transportation development. As globalization increased, cities in South and Southeast Asia grew to accommodate needs.69 In conjunction with globalization came major improvements to transportation, which allowed for increased shipments to and from Southeast Asian countries. These new larger cities and the subsequent increase in people coming and going provided an optimal environment for dengue to spread.70 The population of urban centers increased by 130 million in South Asia between 2001 and 2011. It is expected that this trend will continue, and urban populations will increase by 250 million by the year 2030.71
Public transportation has been identified as a significant contributor to dengue spread. A 2022 study in Bangkok, Thailand, aimed to identify the primary causes of areas of high dengue prevalence. Multiple potential contributing factors were put in a statistical analysis and evaluated for the percentage that they explained the variation in the mean number of dengue cases. When public transportation was included as a factor for statistical analysis along with other contributing factors, 92% of the variance in the mean number of dengue cases was explained, compared with only 61% of the variance explained without including transportation. This means that public transportation was a statistically significant factor in determining what caused the spread of dengue in this region.72 The public transportation system of Bangkok includes above and below-ground trains, buses, and ferry boats.73 The advancements of the transportation system itself contributed to the spread of dengue in that it allowed for the virusAny of a large group of submicroscopic infectious agents that are usually regarded as nonliving, extremely complex molecules that typically contain a protein coat surrounding an RNA or DNA core of genetic material but no semipermeable membrane, that is capable of growth and multiplication only in living cells, and that cause various important diseases in humans, animals, and plants.12 to not be confined to one location.74
Air travel has had significant impacts on dengue transmission. Between the years 1950 and 2011, the annual number of people traveling by airplane increased from 68.5 million to 2.75 billion.75 A 2017 study investigating the influence of air travel on dengue transmission found that air travel was a statistically significant driver of the spreading of the disease. When compared with many other potential influencers for the spatial spread of dengue, such as socioeconomic characteristics and maritime travel, air travel passenger flow was by far the most influential factor.76 Air travel makes it possible for dengue to be more easily transported from disease burdenThe total, cumulative consequences of a defined disease or a range of harmful diseases with respect to disabilities in a community.1 areas to areas of lower incidence.77
Stagnant Water via Poor Waste Management
Stagnant water in close proximity to communities in South and Southeast Asia has contributed to the spread of dengue fever through poor waste management practices, which allows the breeding of aedes aegypti mosquitoes to happen more rapidly.78 The rapid increase of populations in urban centers of South and Southeast Asia has resulted in the inadequate cleanup of sewage.79 Modern objects such as rubber tires that have been discarded and left on the streets in urban centers have become excellent sites for mosquito reproduction.80 In Laos and Thailand, water for household use is often stored in open, dirty containers such as jars and tanks, increasing the risk of dengue in these areas.81
Photo by Kelly
The practice of open dumping, or depositing waste in areas that are not official landfills, is common throughout Southeast Asia.82 In fact, open dumping is the most common form of waste management in 79% of cities in South Asia and 64% of cities in Southeast Asia.83 Out of the 50 largest open dump sites in the world, 17 are in Asia.84 In a 2020 study in Brazil (a country with similarly high rates of dengue), poor waste management was linked with a higher incidence of dengue.85
The second problem with waste management systems in South and Southeast Asia is landfills. Landfills are the most common method of waste management to treat solid waste in South and Southeast Asia, and while they can be effective in dumping mass quantities of waste, the distance from urban centers where waste is generated to the sanitary landfills makes unofficial, open, and unsanitary dumping areas in cities the more common choice.86 Although statistics that demonstrate that areas closer to unofficial landfills lead to higher rates of dengue are lacking, these unsanitary dumping areas create landfills that lack a system to manage leachate (water that builds up by leaking out of solid waste), which creates ideal breeding space for dengue-carrying insects.87
A third issue with the waste management of South and Southeast Asia is that wet waste is often not separated from dry waste.88 A combination of leachate and mixed wet and dry waste in city streets provides a better environment for the aedes aegypti mosquito to reproduce. Additionally, the convenience of modern inventions involving plastic waste contributes to increased trash, thus providing more places for stagnant water to exist.89 When female aedes aegypti mosquitoes lay eggs on the walls of these containers, the eggs can survive for up to 8 months if the containers are not cleaned properly. When rain or other water fills the container, they hatch immediately.90 One interventional study demonstrated that 21% of observed storm drains with standing water contained aedes aegypti larvae, but once the water was removed, no larvae were found.91 Plastic products makeup between 10–18% of the total waste, depending on the country, in Southeast Asia.92
Several issues exist with the waste management system in South and Southeast Asia, allowing for the problem of dirty stagnant water to worsen. First, populations in South and Southeast Asian countries are growing rapidly. With increased populations and subsequent economic growth, waste has increased substantially.93 In many larger cities, poor urban planning has resulted in distances between waste pick-up sites and disposal sites being too far for trash collection services to travel.94 This results in a buildup of waste in areas other than dumps, such as secondary storage points provided by community resources, but these are often emptied too infrequently.95 In South Asia, the rate of trash that is systematically collected is only 44%, leaving the rest of the trash to be dealt with by individual citizens.96 In Manila, Philippines, 15% of the 7,000 metric tons of generated waste is not collected by a government-implemented and funded system and therefore ends up being dumped in the streets or in waterways.97
In 2016, 1.2 billion tons of waste were produced by Asia. That number is expected to increase to 1.5 billion tons by the year 2030.98 The greatest increase in waste generation by 2025 is predicted in Thailand, Malaysia, the Philippines, and Indonesia. In Thailand, waste generation will increase from about 1.1 kg per capita per day to about 1.6 kg, in Malaysia from 0.8 kg to 1.4 kg, in the Philippines from 0.5 kg to 0.8 kg, and in Indonesia from 0.7 kg to 1.0 kg.99 As more waste accumulates without improvements to collect and dispose of the waste properly, more stagnant water will build up near households and dwellings, allowing for mosquito reproduction to happen faster.
Ineffective Prevention Strategies
Ineffective prevention strategies and lack of implementation of successful prevention strategies in South and Southeast Asia have contributed to the perpetuation of dengue fever in this region. Struggling economies in many of these countries have inhibited effective prevention.100 As a result of limited government budgets, funds must often be split between the prevention of dengue and treatment, which results in poorer quality interventions.101 A common preventative technique in countries with high rates of dengue is the use of insecticide, and while it has proven to be effective in dengue reduction for a time, overuse can cause a greater problem of insecticide resistance in mosquitoes. When mosquitoes are exposed to insecticides frequently, they evolve and adapt to resist them.102 In Indonesia, one insecticide used for dengue treatment was observed to cause only 0–1.33% mortality in aedes aegypti mosquitoes, demonstrating that the mosquitoes had become significantly resistant to the insecticide. In Malaysia, a study demonstrated that 75% of aedes aegypti mosquitoes were resistant to 1 type of insecticide. In Vietnam, the mosquitoes were resistant to 5 different types of insecticides.103
Poor disease monitoring, reporting, and surveillance also contribute to the increased prevalence of the disease.104 In many countries disease burdenThe total, cumulative consequences of a defined disease or a range of harmful diseases with respect to disabilities in a community.1 to dengue, an epidemic is frequently not recognized until it is well underway because it is not well-monitored.105 One study estimated that the actual burden of dengue is 282 times higher than what is reported.106 Poor surveillance of the disease by the government means that attempts to control mosquitoes may be less effective because the disease is already spreading rapidly by the time interventions begin. In addition, during epidemics, hospitals become overburdened with too many patients to care for, and transmission of the disease cannot be stopped quickly enough.107 In 2021 in Dhaka, Bangladesh, cases became so high that a hospital that had 16 beds designated for dengue patients admitted 59 patients suspected to have dengue.108
A case study in India demonstrates the insufficient surveillance methods of dengue in South and Southeast Asia. The 2020 study conducted in the Hyderabad region of India concluded that only 2–8% of dengue cases in private hospitals are reported to the proper agencies. This is due, at least in part, to the lack of technology to confirm dengue cases, paper-based reporting, and inefficient systems.109 By comparison, during the outbreak of ebola, another mosquito-transmitted disease, in 2014–2016 in Africa, disease surveillance was significantly improved by practices implemented by the Centers for Disease Control. These methods included contact tracing, case-finding, national alert systems, and walk-in availability for treatment centers. These interventions demonstrated ways to effectively improve surveillance of mosquito-borne illness in resource-poor areas.110
It is important to note that no vaccineA preparation of killed microorganisms, living attenuated organisms, or living fully virulent organisms that are administered to produce or artificially increase immunity to a particular disease.10 exists for individuals who have never been infected. As a result, those at higher risk of contracting the disease cannot act proactively and protect themselves through vaccination. If they contract the disease, they must wait for natural immunity to help protect them in the future. A vaccine to prevent dengue in individuals who have never contracted the virusAny of a large group of submicroscopic infectious agents that are usually regarded as nonliving, extremely complex molecules that typically contain a protein coat surrounding an RNA or DNA core of genetic material but no semipermeable membrane, that is capable of growth and multiplication only in living cells, and that cause various important diseases in humans, animals, and plants.12 is in the process of being developed; as of 2020, there were 6 potential vaccines in various stages of development. Two trial vaccines are in stage 3, 1 trial vaccine is in stage 2, and 3 trial vaccines are in stage 1.111 In stage 1, vaccines are tested among small human samples; in stage 2, human trials are expanded to include people more likely to contract the disease; in stage 3, human testing is further expanded to trials of thousands of subjects.112 In 2015, a vaccine for dengue called Dengvaxia went on the market, but it is only effective in individuals who have previously had dengue fever. In individuals who have never been infected, a 2017 retroactive study demonstrated that they would be more likely to become infected with the more severe hemorrhagic dengue after taking the vaccine; however, statistics for how much more likely individuals were to become infected were not available.113 Screening is recommended by the World Health Organization prior to vaccination to ensure that patients are only vaccinated if they have been previously infected and only in areas disease burdenThe total, cumulative consequences of a defined disease or a range of harmful diseases with respect to disabilities in a community.1 to dengue.114
Other methods for preventing dengue include personal actions to avoid being bitten by mosquitoes, such as using screens, keeping homes cool, keeping arms and legs covered, and using mosquito repellant like DEET. Trapping mosquitoes can also be used to avoid the spread of disease, which will be discussed more thoroughly in a later section.115
Consequences
Morbidity and Mortality
Dengue fever causes physical health complications and significant physical suffering because of the nature of the disease’s progression. Some of the most common symptoms of dengue fever include headache, muscle and bone pain, nausea and vomiting, and rash.116 One patient who contracted dengue explained her pain as indescribable, with a headache so painful she did not want to move her eyes.117 The magnitude of this pain would make a person incapable of continuing normal daily activities such as work, school, and social activities.
Severe dengue occurs in only about 1 in 20 (5%) of symptomatic cases, but its symptoms are a large contributor to dengue-based morbidityThe incidence of disease: the rate of illness (as in a specified population or group).5.118 The most critical health problem caused by severe dengue fever is the damage done to the blood vessels in hemorraghic feverAny of a diverse group of virus diseases (such as Lassa fever and Ebola) that are usually transmitted by arthropods or rodents and are characterized by a sudden onset, fever, aching, bleeding in the internal organs, petechiae, and shock.4, characterized by the bursting of blood vessels under the skin.119 Blood platelets are cells that form clots, so when platelets are low, which is caused by dengue, and vessels are bursting, internal bleeding will ensue. In some severe cases, the disease can escalate to what is referred to as dengue shock syndrome (DSS), characterized by blood pressure dropping to dangerously low levels.120 Symptoms of DSS include vomiting, extreme stomach pain, difficulty breathing, cold and clammy skin, blood in excretions, and bleeding under the skin.121 If left untreated, dengue can result in death in 13% of cases.122 However, when treated early, mortality rates are less than 1%.123
The impacts of dengue extend beyond merely the symptoms that are caused by the disease itself. These impacts include symptoms lasting beyond the acute period of illness or the time period when symptoms directly related to the disease are present. These lasting effects include myalgiaPain in one or more muscles.7, headache, and fatigue.124 The exact percentage of individuals who suffer from these post-acute symptoms varies from study to study, but it appears that by 3–4 months after the initial infection, most symptoms will have subsided.125 Additionally, in pregnancy specifically, the rates of premature births increase when mothers are infected with dengue at any point during their pregnancy.126 In a retrospective study of pregnant dengue patients, 41.5% of patients were at risk for premature labor, and actual premature births occurred in 19.6% of patients.127
The likelihood of having severe dengue is increased when individuals have concurrent diagnoses of some chronic illnesses, which are referred to as comorbidities. In a systematic literature search, researchers found that the prevalence of heart disease and diabetes were 3–4 times more common in cases of severe dengue than in less severe cases. Asthma and obesity were 1.5 times more common in more severe cases than in less severe ones.128 These findings indicate that individuals who have some chronic illnesses will be more likely to develop severe symptoms if they become infected with the dengue virusAny of a large group of submicroscopic infectious agents that are usually regarded as nonliving, extremely complex molecules that typically contain a protein coat surrounding an RNA or DNA core of genetic material but no semipermeable membrane, that is capable of growth and multiplication only in living cells, and that cause various important diseases in humans, animals, and plants.12.
Economic Burden
Dengue fever contributes to an increased economic burden on households and communities through direct and indirect costs associated with the disease. The worldwide disease burdenThe total, cumulative consequences of a defined disease or a range of harmful diseases with respect to disabilities in a community.1 cost has been estimated to be anywhere between $8.9–39.3 billion (USD); such a large difference is likely due to poor record-keeping in developing countries where the disease is prevalent.129, 130 Each symptomatic case costs about $414.00.131 In 2016, the annual national burden of dengue was $5.7 billion in India alone, with 14.3% of the total cost from deadly cases despite less than 1% of cases of death causing dengue.132 When considering the economic consequences of a disease, it is significant to consider the economic burden of the disease on healthcare facilities, communities, and infected individuals. Health costs incurred by the healthcare provider include equipment, payment of healthcare workers, transportation to the care facility, treatment there, and testing.133 Healthcare providers may incur costs such as equipment and supplies, drug testing, and the cost of hospital admission. Communities may incur costs, including the loss of tax revenues from individuals who die, loss of revenue from missed work, and impaired tourism due to fear of contracting the disease.134 In a 2022 study regarding the costs of dengue in Sri Lanka, it was determined that hospitals paid an average of $225.65 per hospitalized patient and an average of $733.46 for patients in an intensive care unit.135
Dengue fever has been demonstrated to have a significant impact on individual and household finances due to the cost of seeking care and missed days at work. Costs to the individual seeking care include factors such as insurance co-payment or out-of-pocket payment if they are uninsured, drug costs, and transportation and other travel costs.136 One study in Sri Lanka broke down the estimated costs of some of these aspects of care-seeking. The study estimated that an average of $3.70 was spent on medications, $8.05 on laboratory investigations, and $11.03 was spent on travel.137 Several studies related to the cost of seeking care for dengue in Thailand indicated that the cost per person ranged from $21.00 to $67.00. The average household monthly income in Thailand is around $283.00, with typical household expenditures totaling about $229.00. The typical individual with dengue misses 4–5 days of work, or about $20.00, which results in a loss of 37% of the remaining household income or income available after household expenses. When the work loss is combined with the direct costs of seeking care, a family might spend 81.5% of their remaining household income on seeking care for dengue if only one family member is sick. It is significant that in an infected household, an average of 1.4 people will contract the disease.138 Individuals who have sick family members may have to stay home to take care of them instead of going to work, thus increasing the economic burden on the family.139 Due to the many aspects of dengue fever associated with economical cost, this disease can cause a significant burden on individuals, families, and communities.
Mental Health
Being infected with dengue fever contributes to poor mental health by causing extreme physiological and psychological stress. Due to the physically traumatic nature of the disease, many dengue patients experience psychiatric symptoms such as depression and anxiety during the acute phase of the illness or the period shortly after infection. In a literature review on the psychiatric effects of dengue fever, researchers reported that 60–90% of patients with dengue experienced both anxiety and depression while they were ill.140 One study demonstrated these percentages being significantly lower among pediatric patients during the acute phase of the disease, indicating that 13.3% of children experienced depression and 34.2% experienced anxiety.141 Another study showed that 80–90% of individuals experienced relief from anxiety symptoms after they began to get better, but about 5% continued to experience anxiety 3 months after they were sick.142 While a significant number of dengue patients demonstrate symptoms of anxiety and depression, this is not equivalent to a clinical diagnosis based on the DSM-V criteria, which is the diagnostic criteria for mental health illnesses.
Dengue has also been linked with the development of phobia disorders and post-traumatic stress disorder.143 Another study demonstrated that during the acute phase of the illness, about 90% of patients experienced severe fear of death. This figure decreased when patients began to recover, with only 50% of patients still demonstrating symptoms of the phobia. This study did not state whether patients were diagnosed with less severe or more severe dengue.144 In the same study, 23% of patients had a panic attack during their illness, and 20% of patients needed to be prescribed anxiolytic (anti-anxiety) medications such as benzodiazepines.145 Because physical and psychological health is intrinsically linked, it is unsurprising that dengue patients have such high rates of psychological distress due to the intense physical trauma inflicted on the body of the sufferer in combination with the stress of hospitalization and seeking care.146
Practices
Mosquito Surveillance and Control
Accurately identifying aedes aegypti mosquitoes that carry dengue is an important aspect of controlling dengue incidence in South and Southeast Asia because it will allow early interventions to prevent dengue outbreaks. However, there has been limited implementation of effective practices, and communities have relied on practices that were labor-intensive, and that yielded poor results.147 In a recent experimental study, a research team identified a new, cost-effective method of identifying dengue-carrying aedes aegypti mosquitoes in Malaysia. In this intervention, gravis oviposition sticky (GOS) traps, which are traps designed to catch mosquitoes made from inexpensive and readily-available materials, were placed in various apartments in Malaysia. The trapped mosquitoes are tested for the presence of dengue, and health officials can then be made aware of a potential outbreak. The research team for this project received approval from the University Malaya Medical Center in Malaysia. The experiment was carried out at the Damansara Damai or Petaling Jaya Utara 10 apartments in Selangor, Malaysia. The trial was carried out from October 2018 to March 2020 and was registered through ClinicalTrials.gov.
Impact
A 39.9% reduction in dengue cases was observed in the intervention group that received the GOS traps, compared to an 18.7% reduction in the negative control group, which received only the standard interventions.148 A similar type of trap used in Singapore demonstrated a 36% reduction in dengue cases compared to control groups.149 This indicates that the GOS traps intervention is significantly more effective than the traditional practices of mosquito surveillance and control.
Gaps
Some gaps in the research include that the GOS traps and similar are only cited in this study as having been implemented on a trial basis and only in Malaysia and Singapore. In order to determine the generalizability of this intervention, it would be necessary to implement it in other countries. Because it has only been used on a trial basis, there is potential for sample bias based on the small geographic area tested. National statistics on dengue case reduction are not available because it has not been implemented nationally in Malaysia. Furthermore, the success of the intervention is predicated upon the swift action of governmental health departments once dengue-carrying mosquitoes have been identified. In the case of this study, the successful reduction of dengue cases would not have occurred solely based on the identification of the dengue-carrying mosquitoes if the health department had not also intervened with preventative measures. Furthermore, this study only measured the short-term effects of this intervention (1–2 years), so there is a need for additional research on the long-term effects of the practice.150
Improved Fluid Management
Dr. Lak Kumar, a pediatrician in Sri Lanka, has pioneered a new method of dengue fever treatment specifically for patients with severe dengue hemorraghic feverAny of a diverse group of virus diseases (such as Lassa fever and Ebola) that are usually transmitted by arthropods or rodents and are characterized by a sudden onset, fever, aching, bleeding in the internal organs, petechiae, and shock.4. In Sri Lanka, mortality rates in cases of severe dengue were between 30–40%.151 This is significantly higher than the global average of 1% mortality in cases where treatment is available early.152 Dr. Kumar’s goal was to reduce patient death through improved care strategies and the education of physicians.153 In 2009, Dr. Kumar began an intensive study of all of the pediatric deaths from dengue in the hospital, where he worked on analyzing the medical complications surrounding the deaths. He also worked with dengue experts in Thailand, and together they identified improved fluid management as a key missing factor in dengue treatment.154 Based on his findings, Dr. Kumar worked to revise standards of practice to include improved fluid management. He also established a Dengue High Dependency Unit (DHDU) for patients with severe dengue, continuous blood monitoring, and a 24-hour hotline for individuals in the community to receive answers to questions about dengue. When he was accepted as an Ashoka fellow in 2012, the DHDU had zero dengue fatalities. He has continued to provide training to physicians and other healthcare workers on improved fluid management as an integral part of dengue care and took his work to Pakistan following devastating flooding in 2011, which resulted in what was likely the largest outbreak of dengue fever. He has also worked with the World Health Organization to revise global standards of dengue treatment.155
Impact
In Ragama hospital, where Dr. Kumar practices, there were no reported deaths from dengue from the time of the implementation of the DHDU, blood monitoring system, and physician information program until he was accepted as an Ashoka fellow in 2012.156 In a study that Dr. Kumar co-authored in 2020, he evaluated the efficacy of fluid replacement in 400 patients with severe dengue and concluded that treating severe dengue early with this treatment resulted in zero fatalities from dengue.157
Gaps
One major limitation of Dr. Kumar’s work is that his treatment applies only to cases of severe dengue; treatment of the majority of non-severe dengue is limited primarily to taking acetaminophen and waiting for symptoms to subside. There is also a lack of more current statistical data to demonstrate the efficacy of the intervention.
Preferred Citation: Lubin, Mikaela Daly. “Dengue Fever in South and Southeast Asia.” Ballard Brief. February 2023. www.ballardbrief.byu.edu.
Viewpoints published by Ballard Brief are not necessarily endorsed by BYU or The Church of Jesus Christ of Latter-day Saints